WAMA Diagnostics Switzerland is a company focused on the development of innovative products for laboratory diagnosis
WAMA Diagnostics is a Swiss SME based in Monthey since 2016 with strong Brazilian roots.
The aim of the company is to improve patient’s life through the development of innovative In Vitro Diagnostic (IVD) tests.
The focus is to develop a MULTIPLEX IMMUNOASSAY for Transfusion-Transmissible Infections (TTI) on blood donors. This technology can be applied to different platforms according to application requirements.

Founded in the end of 2015 with the goal to become a center of development of innovative In Vitro Diagnostic (IVD) products.

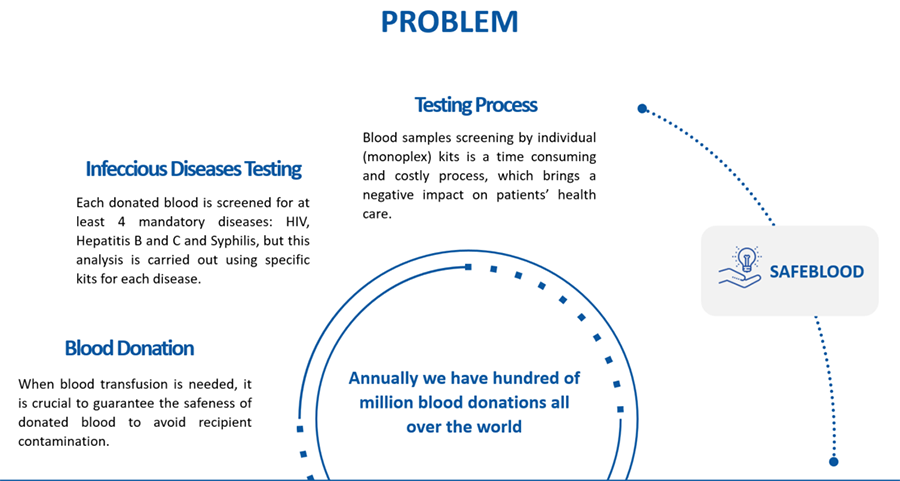
In the past few years WAMA has been working on the development of Safeblood, an innovative in vitro diagnostic multiplex test that allows screening donated blood for bloodborne infectious pathogens.

This new assay performs several tests simultaneously in the same reaction container, which enables higher number of results in a shorter period of time employing lower sample volume.

The company has been financed by Group Maricondi, and several national and European Organizations allowing to build a strong team to run its activities. Recently, it received a €1.7M through Eurostars Program to develop the Safeblood project.

Development of Safeblood is well advanced with the multiplex tests resulting clinical sensitivity minimal of 96,15% (most parameters with 100%).
SOLUTION = SAFEBLOOD


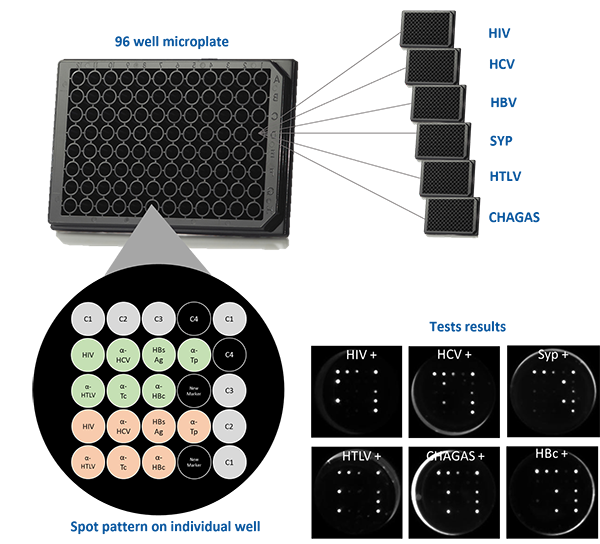
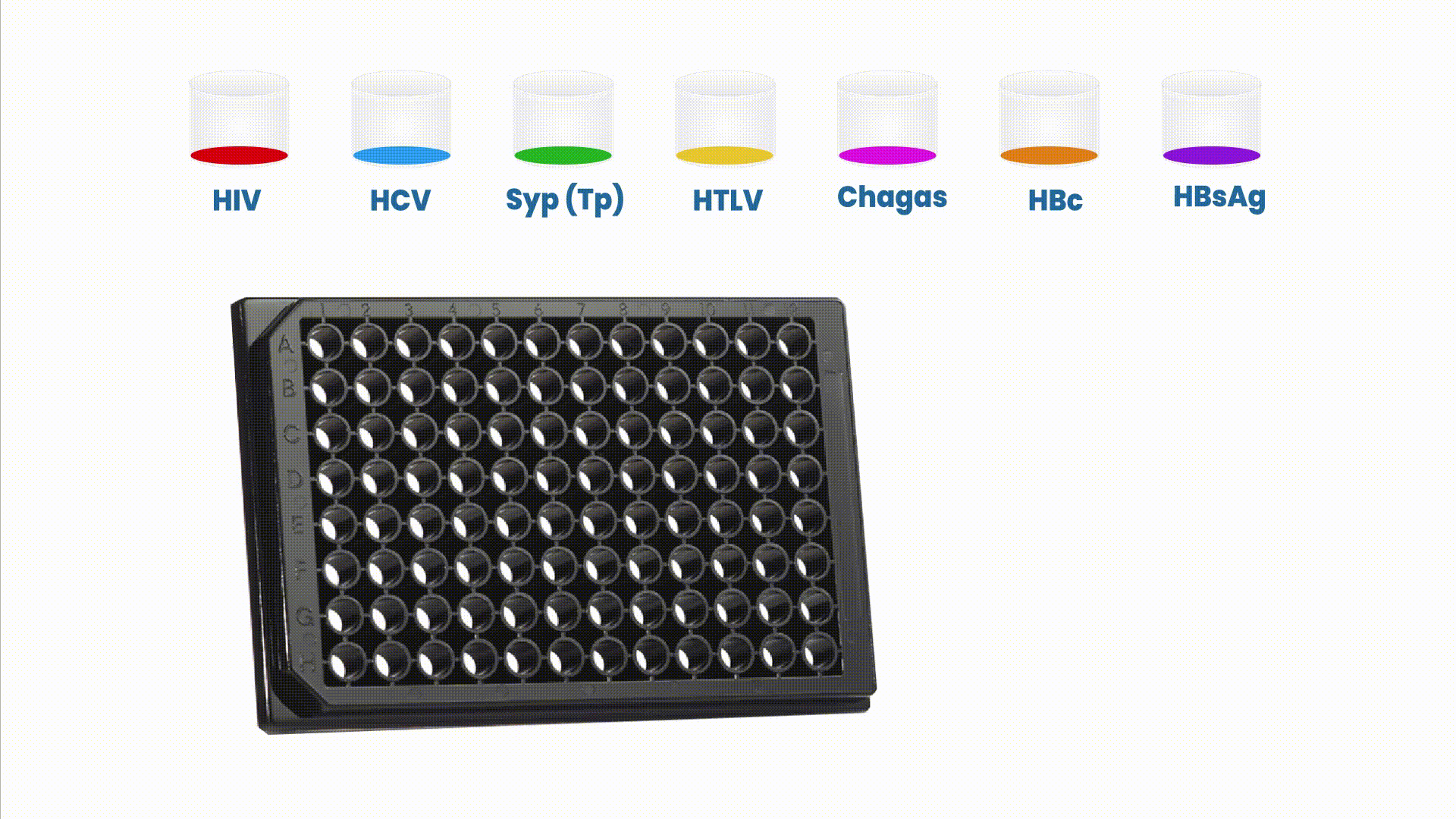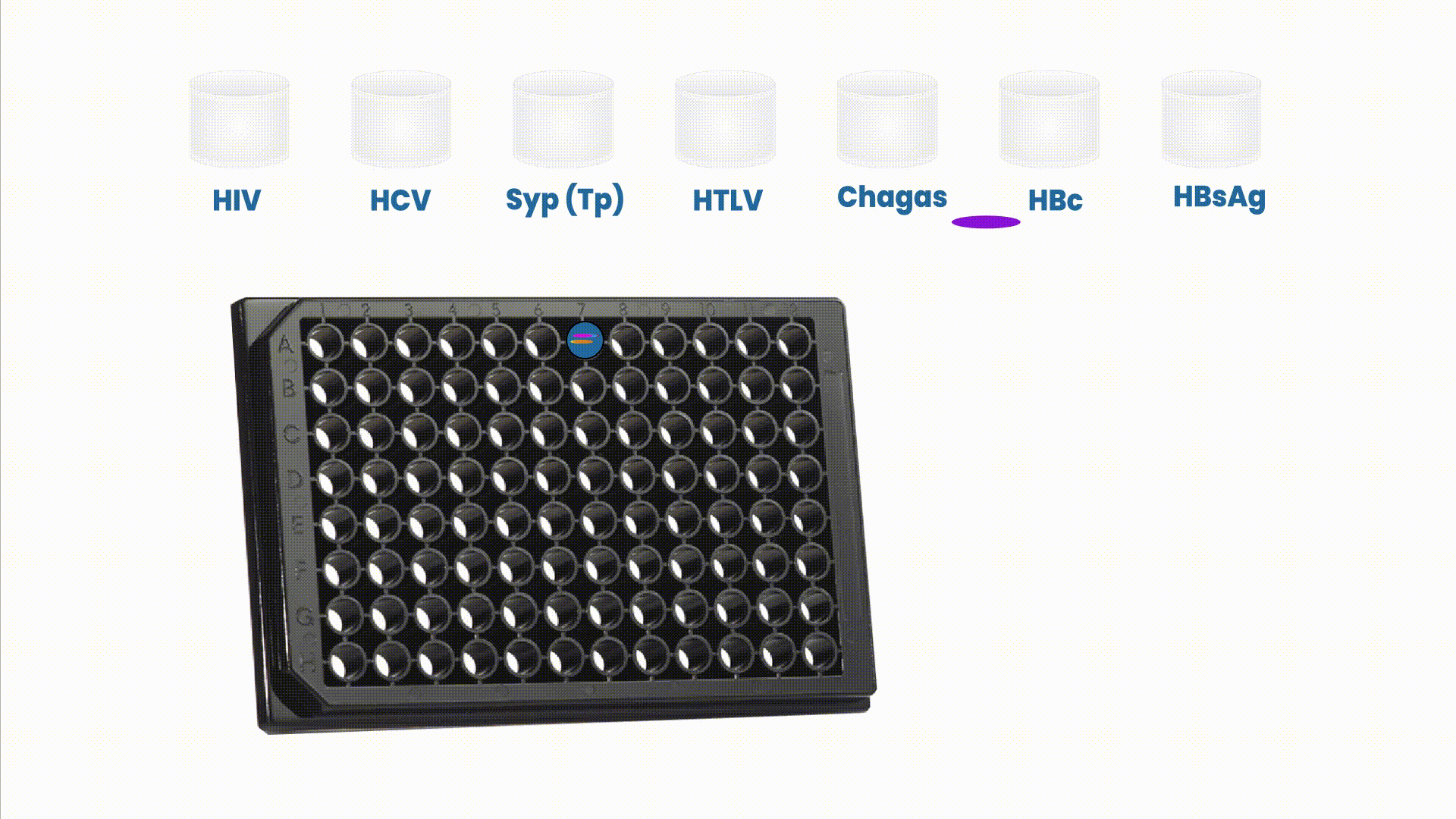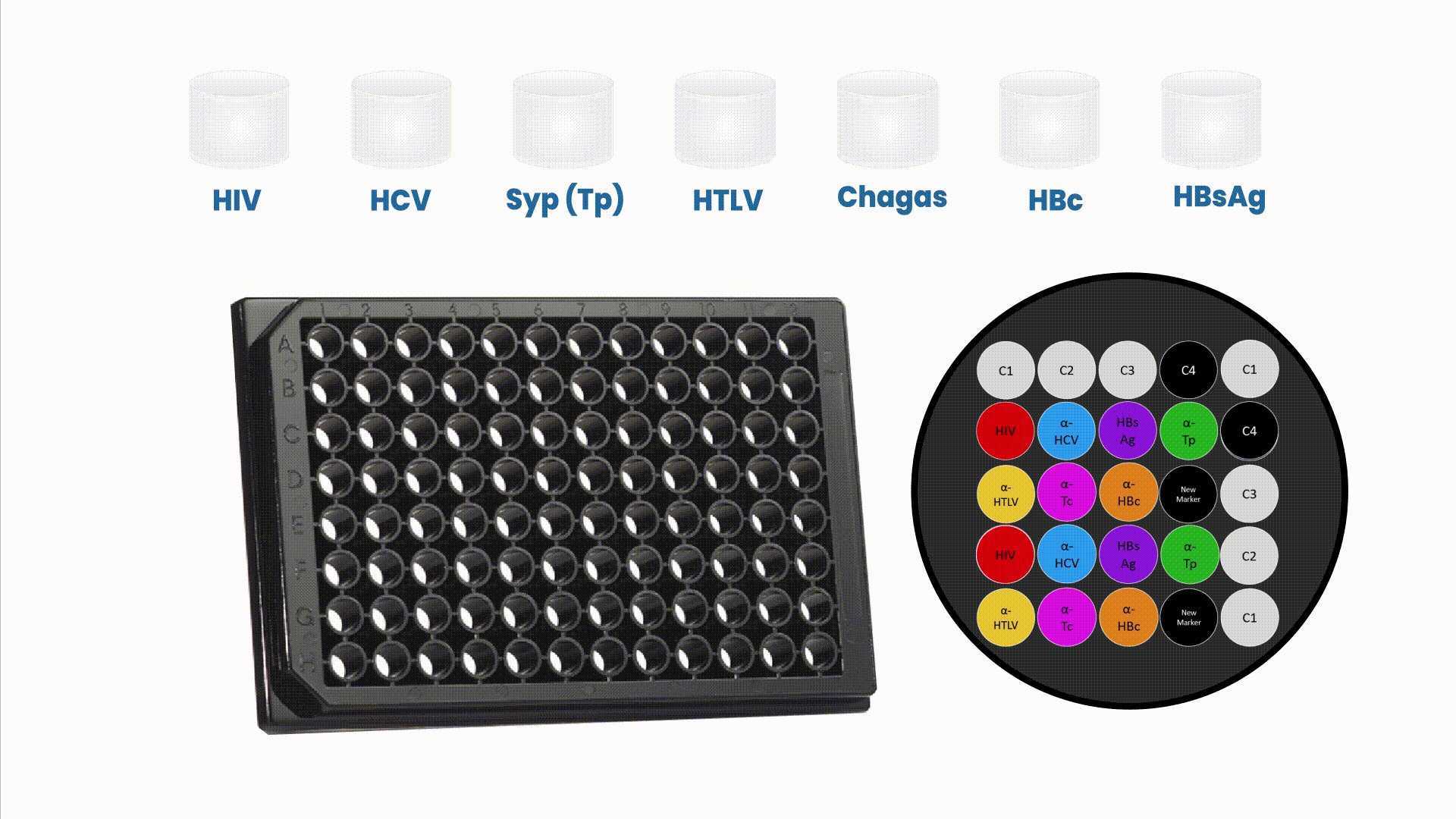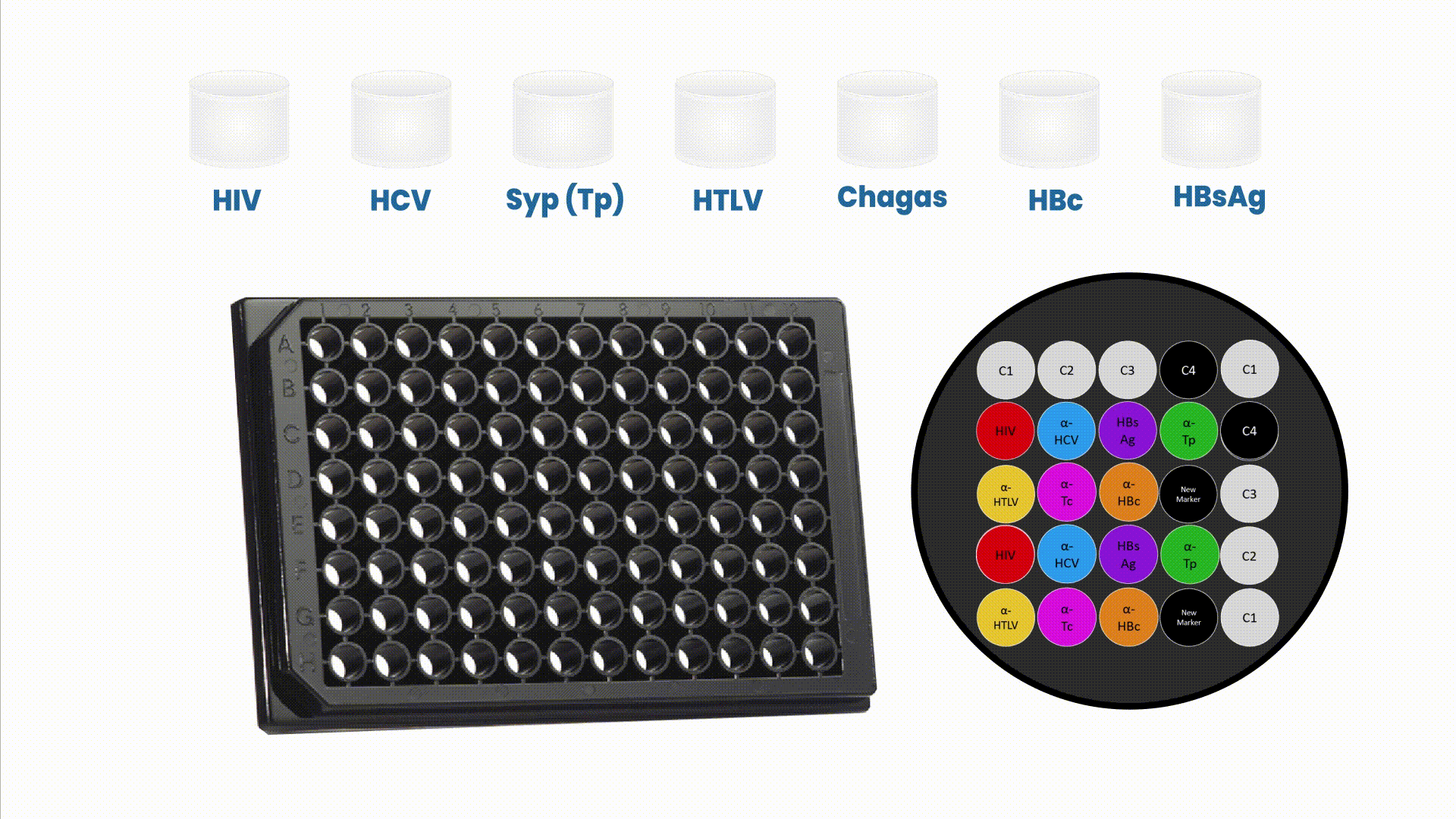
The multiplex assays have the capability of generating multiple and complementary information in a single reaction run to accurately profile patient’s health condition.
Safeblood combines the advantages of the modern microarray technology and its specific readers to the benefits of the well-established ELISA technique and its liquid handling solutions. The integration of referred reader into the ELISA equipment will enable the full automated use of SAFEBLOOD assay in the frame of blood bank laboratories.
This array is composed by positive (C1, C2, and C3) and negative controls (C4). These controls play a critical role to verify if reagents from the assay are working properly, if sample was added to the well and if microplate is read in the right position. Therefore, they allow a stringent control on every patient screening instead of an entire routine.
The Safeblood chemiluminescence immunoassay (CLIA), with such configuration, is able to provide, in a single reaction well, results for 6 different diseases (AIDS , Hepatitis B and C, Syphilis, adult T-cell leukemia/lymphoma (ATL) and Chagas), screening 7 different biomarkers (two biomarkers for Hepatitis B). Therefore, this assay substitutes 7 individual kits by one multiplex at a standard blood bank serology routine.